
We now ask how the electron in a hydrogen atom gets to a higher energy level.
#HYDROGEN LINE EMISSION SPECTRUM SERIES#
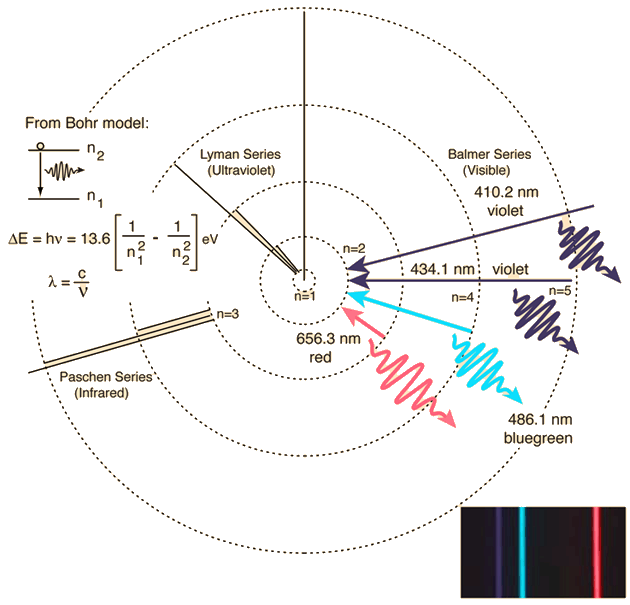
If we change n f to equal 1, we get the lines in the ultraviolet series and when n f = 3, we get the wavelengths in the infrared series for the hydrogen atom. This gives the four wavelengths in the visible region. This is the same constant as in the Balmer formula where n f = 2. Balmer, in 1885, showed the wavelengths in the visible region of the hydrogen atom could be determined by the following equation: The stability of the atom could not be explained by this theory. The electron would spiral into the nucleus within about 10 -10 s. According to classical physics, if an electron was orbiting a nucleus, it would continuously lose energy in the form of electromagnetic radiation. Rutherford’s model stated the atom was made up of a dense positively charged nucleus with negatively charged electrons in the extranuclear region. We will discuss this in more detail later in the study guide. This is due to the electrons in the sodium atoms gaining energy and then emitting the energy as yellow light. If you have ever let food boil over when cooking, you will notice the gas flame will turn a bright yellow-orange. The line spectrum is like a fingerprint and is specific for each element. Each element has its own line spectrum and a line spectrum can be used to identify different elements.

Fireworks are made up of metal salts that when heated will produce light of different colors. When heated, elements will produce line spectra. We see that sodium has two yellow lines that are typical of some street lights. The line spectra below are for lithium and sodium. The colors in the hydrogen emission spectrum correspond to wavelengths of 410.1, 434.1, 486.1, and 656.3 nm. The spectrum is not continuous and only has four different colors. This is called a line spectrum which consists of radiation of specific wavelengths. If hydrogen gas is placed into a discharge tube, and a high voltage is applied, the result is 4 different colored lines. “Europa Rainbow” by Robert Couse-Baker is licensed with CC BY 2.0.


 0 kommentar(er)
0 kommentar(er)
